In a study published in Diabetologia1, researchers compared blood and urine-based biomarker panels to determine if they can outperform current eGFR serum and albumin-to-creatine ratio (ACR) testing. eGFR and ACR results are used to determine renal function in patients with type I diabetes. ACR currently requires a urinary test which patients are less likely to comply with as compared to eGFR serum creatinine test collection.
Nine serum and 13 urine biomarkers were highlighted for testing based on the investigators’ previous discovery research2. The serum markers were KIM-1, Thrombomodulin, SDC1, CD27, TNFR1, MMP8, A1Micro, Clusterin, and CysC. Urine markers included EGF, EGF/MCP-1, IL-6, EGFR, IL-4, Amphiregulin, HB-EGF-like GF, IL-18, Epiregulin, PLGF, MCP-1, IL-8, and MIP-1β. A total of 1629 patient samples were collected from the Scottish Diabetes Research Network Type I Bioresource study.
Sample biomarker testing was completed at Myriad RBM using multiplexed, Luminex technology. Biomarker levels are easily quantified by traditional sandwich immunoassays such as Luminex and ELISA, but KIM-1 levels in serum or plasma are often below LLOQ. Due to this, an ultrasensitive immunoassay for KIM-1 was developed and utilized for the study.
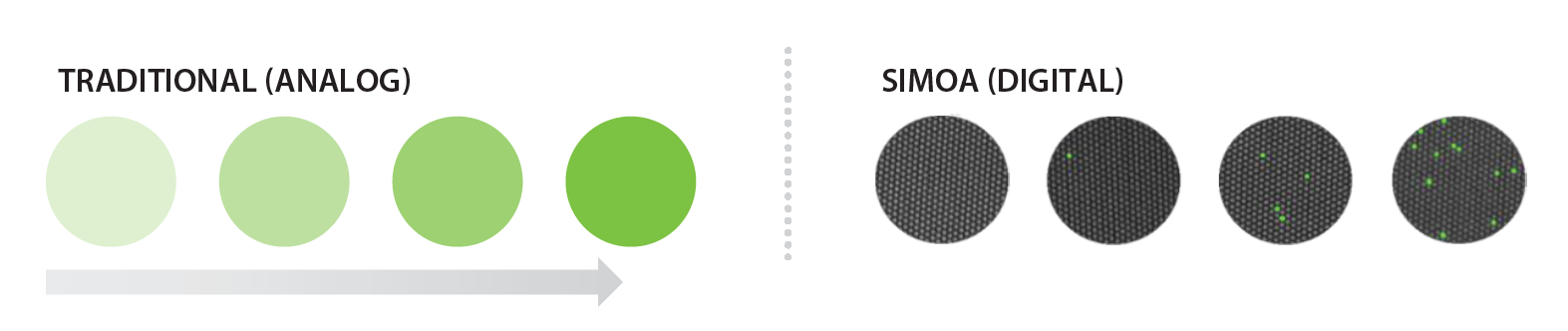
When levels of key protein biomarkers are extremely low in blood plasma or serum, Myriad RBM’s ultrasensitive immunoassay services can quantify such biomarkers with high precision and unprecedented sensitivity. See figure 1 for details on this single molecule array (Simoa) technology.
KIM-1 is an immunoglobulin superfamily cell-surface protein that is highly upregulated on the surface of injured kidney epithelial cells. KIM-1 expression is increased and shed more than any other protein in the injured kidney and is localized predominantly to the apical membrane of the surviving proximal epithelial cells.
Results generated from this research indicated most of the eGFR progression could be predicted through serum KIM-1 Simoa testing in combination with either CD27 or TNFR1. The serum markers were found to outperform traditional ACR measurements. The 13 urine biomarkers selected were not found to be consistently improved predictors compared to ACR. Thus, the need for urinary samples may no longer be necessary.
Figure 1. Left, Analog measurements give increasing intensity as the concentration increases. Right, In contrast, digital measurements are independent of intensity and simply rely on a signal/no signal readout. This approach allows for only a single molecule to be needed to reach the detection limit.
Learn more about Myriad RBM’s ultrasensitive immunoassay development with Simoa Technology.
References:
- Colombo, M., McGurnaghan, S.J., Blackbourn, L.A.K. et al. Comparison of serum and urinary biomarker panels with albumin/creatinine ratio in the prediction of renal function decline in type 1 diabetes. Diabetologia (2020). https://doi.org/10.1007/s00125-019-05081-8
- Colombo M, Valo E, McGurnaghan SJ et al (2019) Biomarker panels associated with progression of renal disease in type 1 diabetes. Diabetologia 62(9):1616–1627. https://doi.org/10.1007/s00125-019-4915-0