Immune Monitoring Solutions for COVID-19 Research
Cytokine Storm Testing for SARS-CoV-2 & COVID-19 Research
Rules-Based Medicine (RBM) is a CLIA certified laboratory providing researchers with reproducible, quantitative immunoassay data from a single drop of blood serum or plasma.
We develop and manufacture our own assays in order to control for lot-to-lot variability and minimize matrix interferences (RF factor, heterophile antibodies).
InflammationMAP® and Immune Markers for COVID-19
Measure 55 key inflammation markers in a cost-effective, and efficient manner from 5 validated multiplex panels covering inflammatory pathways including cytokines, chemokines, vascular proteins and acute-phase reactants. Our biomarker testing services have been used in hundreds of clinical trials and research projects and cited in over 1000 peer reviewed publications.
Blue Text = Multiplex Immunoassays |  = Ultrasensitive Immunoassays
= Ultrasensitive Immunoassays
InflammationMAP®
CORE1 (CytokineMAP®A)
Core2 (CytokineMAP®B)
HMP8
Core4
Additional Markers
2. Interferon beta 3, 4, 5




3. LIGHT 6, 13, 21




4. Neuropilin-1 7, 8
5. Growth/differentiation factor 15 9
6. Interferon gamma Induced Protein 10 11, 14, 19
7. Interleukin-2 receptor alpha 12 8. LAP-TGF-beta 20 9. Carbonic anhydrase 9 1 10. Decorin 1
References
- Distinct patterns of blood cytokines beyond a cytokine storm predict mortality in COVID-19
(2021) Herr C, et al. Journal of Inflammation Research.
https://doi.org/10.2147/JIR.S320685 - GM-CSF-based treatments in COVID-19: reconciling opposing therapeutic approaches
(2020) Lang FM, et al. Nature Reviews Immunology. 20, 507–514 (2020)
https://doi.org/10.1038/s41577-020-0357-7 - Impaired type I interferon activity and inflammatory responses in severe COVID-19 patients.
Hadjadj J, et al. Science 369(6504):718-724.v
Recent coverage of the Hadjadj J, et al. article in BioSpace - Type III interferons disrupt the lung epithelial barrier upon viral recognition.
(2020) Broggi A, et al. Science 369(6504):706-712 - Type I and III interferons disrupt lung epithelial repair during recovery from viral infection
Major J, et al. Science 369(6504):712-717 - Cerecor and Myriad Genetics announce that levels of novel cytokine, LIGHT were highly correlated with disease severity and mortality in COVID-19 ARDS biomarker study
Harrell J and Gleason S. (2020) https://rbm.q2labsolutions.com/2020/05/26/cerecor-and-myriad-genetics-announce-that-levels-of-novel-cytokine-light-were-highly-correlated-with-disease-severity-and-mortality-in-covid-19-ards-biomarker-study/ - Neuropilin-1 is a host factor for SARS-CoV2 infection
(2020) Daly JL, et al. Science.
DOI:10.1126/science.abd307 - Neuropilin-1 facilitates SARS-CoV2 cell entry and infectivity. (2020) Cantuti-Castelvetri L, et al. Science
DOI: 10.1126/science.abd2985 - Growth differentiation factor-15 provides prognostic information superior to established cardiovascular and inflammatory biomarkers in unselected patients hospitalized with COVID-19
(2020) Myhre PL, et al. Circulation
DOI: 10.1161/CIRCULATIONAHA.120.050360 - The role of Interleukin 6 inhibitors in therapy of severe COVID-19
(2020) Nasanov E, et al. Biomedicine & Pharmacotherapy Volume 131, 2020
https://doi.org/10.1016/j.biopha.2020.110698 - A dynamic COVID-19 immune signature includes associations with poor prognosis
(2020) Laing AG, et al. Nature Medicine. 26, 1623–1635 (2020)
https://doi.org/10.1038/s41591-020-1038-6 - The Inhibition of IL-2/IL-2R gives rise to CD8+T cell and lymphocyte decrease through JAK1-STAT5 in critical patients with COVID-19 pneumonia
(2020) Shi H, et al. Cell Death Dis. 2020 Jun 8;11(6):429.
DOI: 10.1038/s41419-020-2636-4 - Biomarkers of COVID-19 and technologies to combat SARS-CoV2
(2020) Zhang L and Guo H. Advances in Biomarker Sciences and Technology. 2020;2:1-23
doi: 10.1016/j.abst.2020.08.0011, - Longitudinal immune profiling reveals key myeloid signatures associated with COVID-19
(2020) Mann ER, et al. Science Immunology 5,5. eabd6197
DOI:10.1126/sciimmunol.abd6197 - Immunophenotyping of COVID-19 and influenza highlights the role of type I interferons in development of severe COVID-19
(2020) Lee LS, et al. Science Immunology. 5, 49, eabd1554
DOI:10.1126/sciimmunol.abd1554 - COVID-19: consider cytokine syndromes and immunosuppression
(2020) Mehta P, et al. The Lancet. 395, 10229. 1033-1034
https://doi.org/10.1016/S0140-6736(20)30628-0 - Serum IgA, IgM, and IgG response in COVID-19
(2020) Ma H, et al. Cellular & Molecular Immunology. 17, 773-775
https://doi.org/10.1038/s41423-020-0474-z - Matrix metalloproteinase 3 as a valuable marker for patients with COVID-19
(2020) Shi S, et al. J Med Virol. 10.1002/jmv.26235
DOI: 10.1002/jmv.26235 - Inhibition of cytokine signaling by ruxolitinib and implications for COVID-19 treatment
(2020) Yeleswarm S, et al. Clinical Immunology. 218, 108517
https://doi.org/10.1016/j.clim.2020.108517 - Interferon gamma, TGF-b1 and RANTES expression in upper airway samples from SARS-CoV2 infected patients
(2020) Clinical Immunology. 220, 108576.
https://doi.org/10.1016/j.clim.2020.108576 - Randomized, double-blind , controlled trial of human anti-LIGHT monoclonal antibody in COVID-19 acute respiratory distress syndrome
(2021) Perlin DS, et al. J Clin Invest. Dec 6;e153173. doi: 10.1172/JCI153173. Online ahead of print.
https://pubmed.ncbi.nlm.nih.gov/34871182/
SARS-CoV-2 T cell activation with TruCulture
Spike Protein tubes
TruCulture is a whole blood collection and culture system for immune monitoring in subjects and has been utilized in hundreds of clinical trials. The SARS-CoV-2 Spike Protein TruCulture tube was designed and developed to be used in clinical trials to:
- Quantify SARS-CoV-2 vaccine-dependent T cell activation.
- Investigate the magnitude and duration of T cell responses post SARS-CoV-2 infection.
Use TruCulture tubes to collect 1 mL of whole blood directly from a patient to immediately start antigen recognition, processing, and presentation in the tube. Each TruCulture tube contains 2 mL of media including the SARS-CoV-2 spike protein antigen. After 48 hours at 37o C, the secreted T cell cytokines IFNγ and IL-2 are quantified by validated immunoassays (RBM’s ultrasensitive immunoassay services).
In comparison to ELISpot utilizing peptide pools, TruCulture recombinant protein antigen recall technology for clinical studies has many advantages:
- More representative of cell mediated immunity with the entire immune system present for the complex interplay of antigen processing, presentation, and T cell recognition
- More sensitive – a higher proportion of viable cells (cell reaction starts immediately upon blood draw)
- Closed sterile system performed with a simple blood draw by a phlebotomist at a trial site with a 37°C degree dry-heat block being the only specialized equipment needed
- More cost-effective
- Utility recently published in
Download the SARS-CoV-2 Spike Protein TruCulture Tubes application note to learn more.
NEW! SARS-CoV-2 Spike Protein TruCulture tube (Delta variant)
In comparison to the original Wuhan-1 SARS-CoV-2 strain, the Delta variant (B.1617.2) Spike protein is defined by upwards of 10 amino acid substitutions in the SARS-CoV-2 spike protein. Four of these substitutions are of concern and are thought to be the primary reason for higher degree of transmission and possibly causing more severe disease1:
- D614G -shared with other highly transmissible Variants of Concern (VOC) (α, β, γ)
- T478K -Receptor-Binding Domain (RBD) region
- L452R -RBD region, higher affinity for angiotensin-converting enzyme 2 (ACE2), more difficult for immune recognition
- P681R -enhances S precursor furin cleavage to active S1/S2 configuration
The remaining six Delta defining Spike protein amino acid changes are:
- G142D
- T19R
- R158G
- D950N
- E156del
- F157del
All ten substitutions and deletions will likely have an important impact on T cell epitope (Class I and II MHC) presentation and recognition by T cells. For previously vaccinated or infected subjects, or new vaccine development studies, the SARS-CoV-2 Delta Variant Spike Protein TruCulture Tube is a powerful addition to the original TruCulture Spike protein tubes for quantifying antigen recall and cell mediated immunity.
Reference:
For other TruCulture Tubes and Stimulants, click here.
Analysis
TruCulture cell pellets can be collected for downstream analyses:
Application Note Download
Download the SARS-CoV-2 Spike Protein TruCulture Tubes application note to learn how TruCulture tubes are used to measure antigen recall responses to the SARS-CoV-2 spike protein.
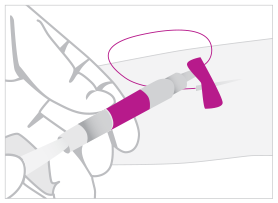

01. COLLECT
Draw 1 mL of blood directly into the TruCulture Tube and break off the plunger.
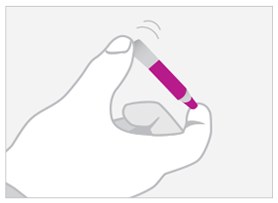

02. MIX
Gently invert tube to mix 3 to 5 times
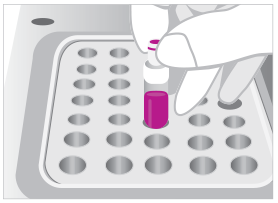

03. INCUBATE
Place tube in 37ºC heat block for up to 24 or 48 hours.
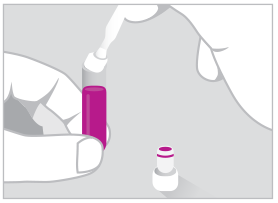

04. SEPARATE
Manually insert valve to separate supernatant from the cells. Collect supernatant and cell layer for downstream analysis.
CONTACT US
- (+01) 512 835 8026
- rbm.info@q2labsolutions.com
- https://rbm.q2labsolutions.com
- 3300 Duval Road
Austin, TX 78759 - Mon to Fri - 8:00am to 6:00pm
Speak to a Scientist
By submitting your information in this form, you agree that your personal information may be stored and processed in any country where we have facilities or service providers, and by using our “Contact Us” page you agree to the transfer of information to countries outside of your country of residence, including to the United States, which may provide for different data protection rules than in your country. The information you submit will be governed by our Privacy Notice.
Multi-Analyte Profile (MAP) Platform
The RBM MAP platform has been built and optimized to help researchers discover and validate biomarker patterns for use in their drug and diagnostic development efforts. Robust multiplexing on the microsphere-based Luminex technology combined with automated liquid handling, delivers more data in less time than other platforms, resulting in comparable data to traditional ELISA assays.
- High throughput multiplexing with automated liquid handling reduces sample volume requirements
- Enhanced accuracy with use of multi-level controls, standard curves, and proprietary blockers
- High quality and reproducibility with stringent quality control parameters for reliable data
We ensure quality for every multiplex, every analyte, every sample, every time.
The RBM platform produces precise and dependable results by utilizing automated liquid handling systems, advanced quality monitoring, validated data reporting processes, and a highly-trained and dedicated staff.
Every analyte included in RBM’s Multi-Analyte Profiles (MAPs) has its own set of calibrators and controls to provide reliable, high quality data. By continuous quality improvement, RBM ensures that customers are satisfied with the products and services they receive.


Other RBM Products
CustomMAP
Our CustomMAP is a flexible option to let you pick specific biomarkers for your research needs. Customize the number of multiplexes to build your CustomMAP.
Speak to a sales representative to learn more about CustomMAP options.
50 sample minimum required. Volume requirements are dependent on the number of multiplexes selected.
Ultrasensitive Immunoassays - Simoa
Looking for trace quantities of markers? Ultrasensitive immunoassays achieve orders-of-magnitude greater sensitivity than conventional immunoassay platforms for when levels of key proteins are extremely low in serum or plasma.


SARS-CoV-2 Vaccine Development Application with TruCulture®
Utilized in hundreds of clinical trials for a variety of applications, TruCulture® is a whole blood collection and culture system for immune monitoring of subjects in clinical trials.
- Detect vaccine-dependent T-cell activation (antigen recall)
- Early safety studies of vaccines and their individual components
- Profile a subject’s functional immune status