Peter H. Schafer, Ying Ye, Lei Wu, Jolanta Kosek, Garth Ringheim, Zhihong Yang, Liangang Liu, Michael Thomas, Maria Palmisano, Rajesh Chopra
Ikaros and Aiolos regulate homeostasis and leukocyte development. Ikaros is expressed in haematopoietic precursors and affects both B and T cell development. Aiolos is only found in pre-B cells and mature peripheral B cells and is necessary for long-lived plasma cells. Polymorphisms in their respective genes (IKZF1 and IKZF3 loci) have been reported to correlate with increased risk for systemic lupus erythematosus (SLE), an autoimmune disease that is characterized by production of autoantibodies. Iberdomide (CC-220) is an oral compound being investigated for treatment of SLE. CC-220 is a high-affinity ligand of cereblon and upon binding leads to degradation of cereblon substrates, Ikaros and Aiolos. The degradation of Ikaros and Aiolos increases T cell production of IL-2, demonstrating potential immune modulatory effects.
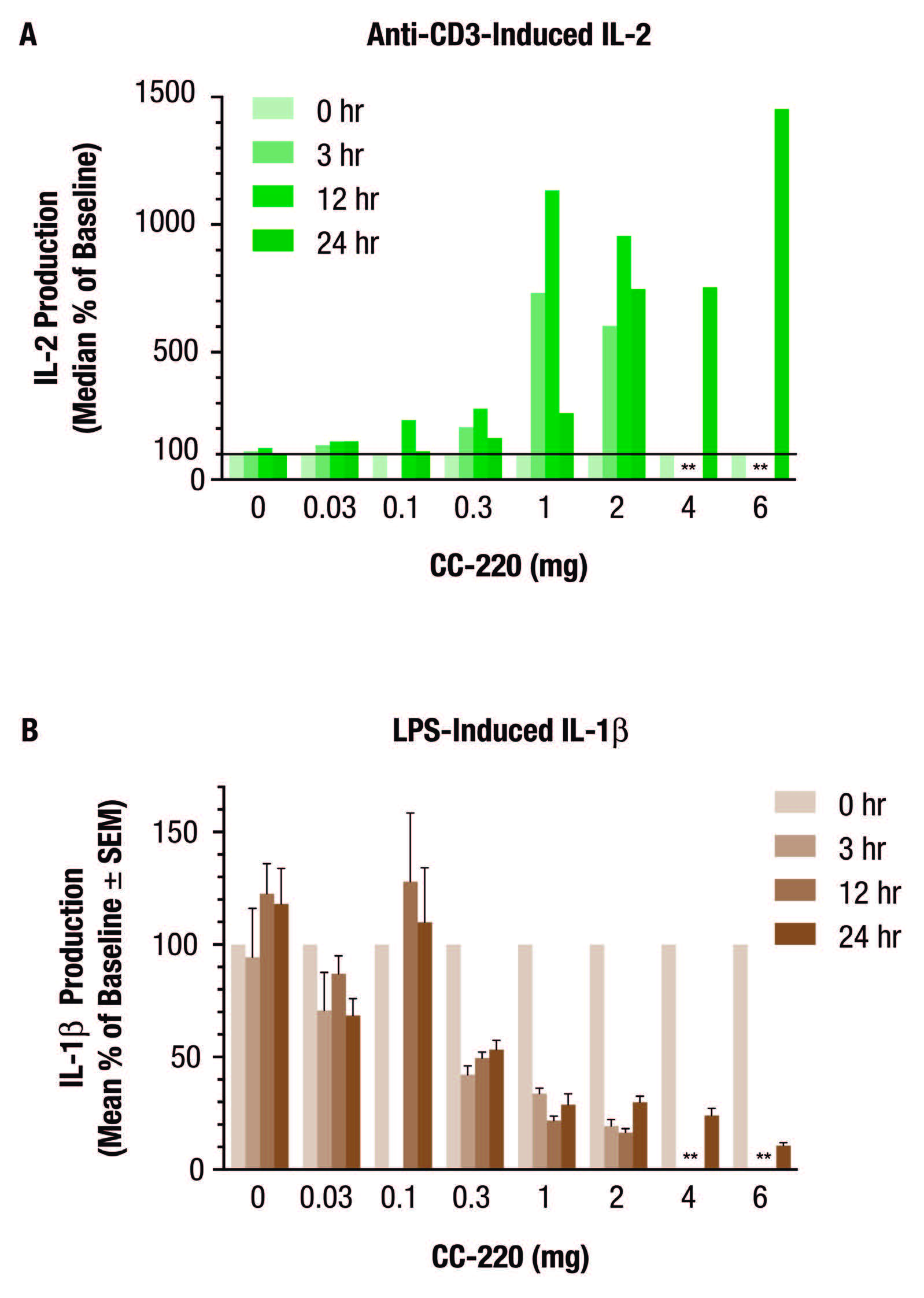
In peripheral blood monocytes (PBMC) isolated from SLE patients, there is overexpression of cereblon, Ikaros, and Aiolos mRNA, and in vitro PBMC cultured with CC-220 decreased anti-dsDNA and anti-phospholipid IgM autoantibody production. Additionally, as part of this study, a phase I double-blind, placebo-controlled, single-ascending dose study was conducted in 56 healthy volunteers. The subjects were randomized into 7 groups, each given one dose of CC-220 at 0 (placebo), 0.3, 0.1, 0.3, 1, 2, 4, and 6 mg. Whole blood was drawn at baseline, day 2, day 3, and day 5 post-dose. Whole blood analysis demonstrated that CC-220 reduced overall B and T cell numbers.
Additional whole blood was also drawn into Myriad RBM’s TruCulture tubes supplemented with anti-CD3 or LPS at 0, 3, 12, and 24hrs post-dose. The supernatants from these stimulated ex vivo whole blood cultures were measured for production of IL-2 and IL-1β respectively. The figure above shows that whole blood collected from donors after CC-220 oral dosing increased IL-2 production under anti-CD3 stimulation. It also demonstrates that CC-220 reduced response to LPS as shown by the decrease in IL-1β. No data was available for the 4 and 6 mg doses at 3 and 12 hour post-dosing. The authors concluded that the various effects of iberdomide on the human immune response indicate its potential to treat autoimmune diseases that are characterized by autoantibody production and inflammation. A phase II clinical trial with iberdomide in SLE patients is ongoing. This study is an excellent example of the clinical utility of TruCulture in analyzing pharmacodynamics in a phase I clinical trial.