Martin Andersen, Mikael Boesen, Karen Ellegaard, Kalle Söderström, Niels H. Søe, Pieter Spee, Ulrik G. W. Mørch, Søren Torp-Pedersen, Else M. Bartels, Bente Danneskiold-Samsøe, Lars Karlsson, Henning Bliddal
The use of biologic disease modifying anti-rheumatic drugs (bDMARDs) significantly changed the treatment of rheumatoid arthritis (RA), however only 15 percent of these patients ever achieve remission. Thus, predicting patient responses to treatment and achieving disease control remains great challenges in RA. One possible solution put forth by the authors is the use of explants, in vitro culturing, of synovial tissue. This study examined the effects of bDMARDs on baseline RA synovial explants production of IL-6 with each individual’s clinical response to the same bDMARDs.
Twenty RA patients were recruited, and a synovectomy, of up to 2 joints, was performed within 24 hours of baseline examination (DAS28CRP, CDUS, and 3 Tesla MRI). Synovial explants (2mg/well in 96 well plates) were cultured with bovine bone slices in RPMI 1640 with 10% FBS, 2% human serum, penicillin and streptomycin. Supernatants were collected prior to bDMARD or isotype control treatment (72hrs) and 2 weeks after antibody addition. Levels of MCP-1, IL-6, and MIP-1b from explant supernatants were analyzed by Myriad RBM HumanMAP technology. Fold change of cytokine production was calculated as a ratio of 2 weeks/72hrs. The explants supernatant IL-6 fold changes were correlated to each patient’s response to bDMARDs clinical therapy.
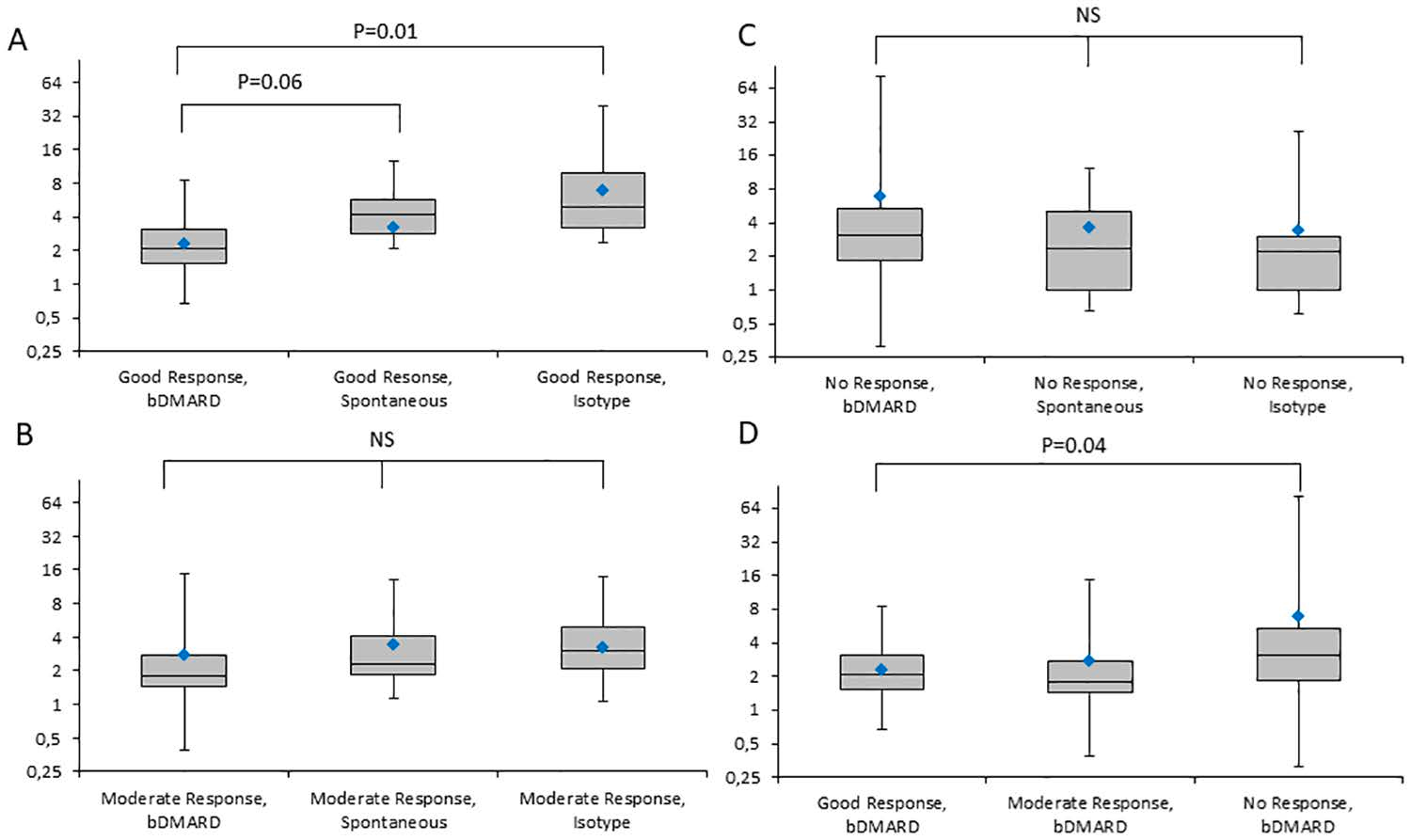
Figure A, B, and C depicts explant IL-6 fold change grouped by clinical response. The figures compared explant cultures treated with bDMARDs, non-treated (spontaneous release), or treated with isotype controls. In patients with good response to clinical therapy, explant supernatants has a significant decrease in IL-6 compared to non-treated and isotype controls. In moderate and no response patient cohorts, the explant cultures with bDMARDs did not show any suppression of IL-6, suggesting that the explant model may provide RA disease activity information. Figure D compares IL-6 levels in relation to the clinical response to therapy, demonstrating that explants from patients with good responses have significantly lower IL-6 compared to explants from patients with no response to therapy. This study demonstrates that Myriad RBM’s HumanMAP analysis of IL-6 production from explant supernatants may aid in predicting a patient’s clinical response to therapy.